Securing a drug license is not merely a legal formality but an indispensable cornerstone of public health. In India, the pharmacy sector is the lifeline for the healthcare business. As the demand for quality healthcare continues to surge, the significance of a well-regulated pharmacy industry cannot be overstated.
Considering the numerous incidents of misuse that have occurred the government imposes strict regulations for the production and distribution of drugs and medicine. Thus ensuring the need for a drug license when engaging in this business.
To sell drugs in India, a Drug license must be granted by the government or State Drugs Standard Control Organization (SDSCO).
In this article, we will explore the different types of licenses issued by the SDSCO furthermore the essential steps their prerequisites, and the application procedure involved in obtaining a drug license, in order to start a retail pharmacy.
Drug Regulation In India
In India, there are two major regulatory bodies for drug regulation.
- Central Drugs Standard Control Organization(CDSCO)
- The regulatory body grants licenses for specific specialized categories of critical Drugs such as blood and blood products, I. V. Fluids, and Vaccines. To start a pharmacy franchise you need to get a drug license approved by this organization.
- State Drugs Standard Control Organization (SDSCO)
- The responsibility for issuing licenses to pharmacists, wholesalers, and retailers, as well as the complete drug manufacturing to the sales process, falls under the jurisdiction of the Drugs and Cosmetics Act, of 1940, and is regulated by SDSCO.
- This act also covers the licensing of importers of drugs, cosmetics, Ayurvedic, Siddha, and Unani medicines.
Drug license in India is area explicit. So pharmacy units that operate in multiple states must obtain drug licenses for each state in which they conduct business.
Types Of Drug Licenses Issued
According to the type of pharmaceutical business you are associated with you should have any of the following licenses.
- Manufacturing Licence
- License issued to entities for manufacturing drugs including allopathic, homeopathy medicines, and cosmetics to sell in India. The SDSCO officials will issue a license after inspecting the manufacturing facility.
- Wholesale License
- Individuals or firms operating a wholesale drug business require a wholesale drug license to distribute drugs to retailers or hospitals.
- Retail Pharmacy License
- The license acquired by retailers enables them to market drugs or cosmetics intended for end-user consumption. Required for retailers involved in pharmaceuticals, cosmetics, stand-alone pharmacists, ayurvedic shops, and similar businesses. To obtain this license, the applicant must submit an application to the SDSCO along with the required documents and fees,
- Loan license
- The type of license granted to an applicant who does not have their own manufacturing unit but plans to utilize a manufacturing facility owned by a different licensee.
- Multi-drug-licence
- The type of license required for pharmacies that have multiple units operating under the same name across different states.
- Import license
- Licenses are issued for a specific duration to entities engaged in the import of drugs into India. Including those importing products for drug manufacturing and other businesses involved in drug importation.
Prerequisites For Obtaining A Pharmacy License
- Infrastructure Requirements
- To obtain a license, most importantly a pharmacy that operates as both a retail and wholesale business must have a pharmacy unit of at least 15 square meters. For a retail pharmacy or medical shop, a minimum area of 10 square meters is required. A commercial building, with an electricity supply, and no direct sunlight.
- Storage requirements
- It is mandatory to have a refrigerator and an air conditioner in the uni. Since some medicines and vaccines must be stored in low temperatures storage requirements should be met.
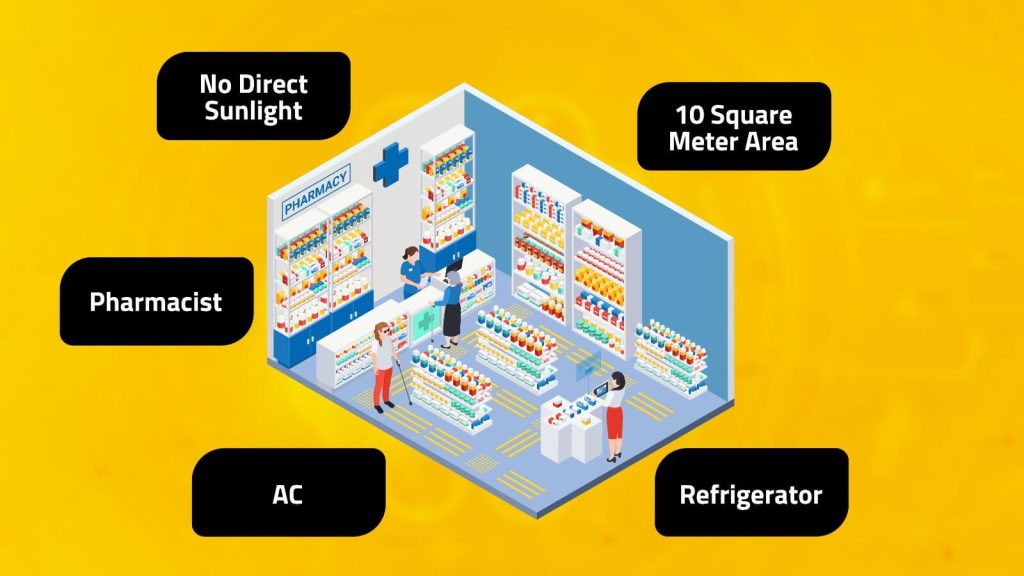
- Technical/Staff Requirements
- A registered pharmacist must be present during the pharmacy’s working hours to sell drugs in a retail pharmacy. Similarly, a registered pharmacist must also be present in a wholesale store.
- However, in a wholesale store, the sale of drugs can be carried out by,
- A person who has a graduate degree with one year of experience.
- A person who has completed SSLC and has four years of experience approved by the drug control department.
- Building
- The pharmacy unit should be a commercial building with the following rent agreement.
- The rent agreement should be in the proprietor’s name if the pharmacy unit is under proprietorship.
- If the pharmacy unit is under a partnership rent agreement should be in the name of the managing partner on whose behalf the drug license is obtained.
- The pharmacy unit should be a commercial building with the following rent agreement.
Drug License Application Procedure
The applicant must apply to the respective drug controlling authority based on the license he/she requires as each drug controlling authority (SDSCO, CDSCO, or Ayush) is responsible for issuing different licenses
- Step 1 – Filing application
- Obtain the necessary drug license application form online from the respective drug controlling authority website. (https://dc.kerala.gov.in/en/online-licensing/) The applicant must fill out all the necessary drug license application forms.
- Step 2 – Attach documents
- Upload your documents along with the filled application form and applicable fees. Applicant should make sure all documents are updated
- Step 3 – Drug inspection
- Upon receiving the application, the relevant authority will inspect your company’s premises to verify the authenticity of the information provided in the application form.
- Step 4 – License issued
- If the inspection is successful you will be issued a drug license to operate your business.

List Of Essential Documents
- Duly filled application form
- Fee receipt or copy of challan as proof of payment
- Documents regarding the company premise – ownership document of property / rental agreement
- ID proof of every partner along with a copy of the partnership deed.
- Applicant’s qualification certificate
- Registration certificate issued by pharmacy council
- Education qualification certificates
- Appointment letter
- Affidavit of pharmacist
- Building/shop Ownership certificate
- Copies of the layout plan
- Proof regarding the availability of storage space and necessary electrical appliances
- Copy of Constitution of the entity, Memorandum of Association (MOA), Articles of Association (AOA) for a company, partnership deed, LLP agreement in case of partnership and LLP.
- Copy of board resolution permitting a company to obtain a license
- Cover letter of the applicant with the name, signature, and designation of the applicant and the intent of the application
- Details of the staff and the registered personnel.
- Building tax receipt
- Corporation/ panchayat license
List Of Application Forms required
| SL.No. | Form | Requirement for license |
|---|---|---|
| 1 | Form 19 | Application for grant or renewal of a license to sell, stock, exhibit or offer for sale, or distribute drugs apart from those specified in Schedule X. |
| 2 | Form 19A | Application for granting or renewing a restricted license to sell, stock, exhibit or offer for sale, or distribute drugs by retail via dealers who don’t engage the service of a registered pharmacist. |
| 3 | Form 19B | Application for license to sell, stock or exhibit or offer for sale, or distribute Homoeopathic Medicines. |
| 4 | Form 19C | Application for grant or renewal of a [license to sell, stock, exhibit, or offer for sale, or distribute] drugs specified in Schedule X. |
| 5 | Form 20 | Application for license to sell, stock or exhibit or offer for sale, or distribute drugs by retail other than those specified in [Schedules C, C(1) and X]. |
| 6 | Form 20A | Application for license to sell restricted, stock or exhibit or offer for sale, or distribute] drugs by retail other than those specified in [Schedules C, C (1) and X] for dealers who do not engage the services of a registered pharmacist. |
| 7 | Form 20B | Application for license to sell, stock or exhibit, or offer for sale, or distribute by wholesale, drugs other than those specified in [Schedules C, C(I) and X]. |
| 8 | Form 20C | Application for license to sell, stock or exhibit or offer for sale, or distribute Homoeopathic medicines by retail. |
| 9 | Form 20D | Application for license to sell, stock or exhibit or offer for sale, or distribute Homoeopathic medicines by wholesale. |
| 10 | Form 20E | Application for certificate of renewal of Licence to sell, stock or exhibit or offer for sale, or distribute Homoeopathic medicines. |
| 11 | Form 20F | Application for the license to sell, stock or exhibit for sale or distribute by retail drugs specified in Schedule X. |
| 12 | Form 20G | Application for the license to sell, stock or exhibit or offer for sale, or distribute wholesale drugs specified in Schedule X. |
| 13 | Form 21C | Application for certificate of renewal of the license to sell, stock or exhibit or offer for sale, or distribute drugs. |
| 14 | Form 24A | Application for either the grant of a loan license or renewal of a loan license to manufacture for sale or distribution of drugs other than those specified in Schedule C, C (1) and X. |
| 15 | Form 24B | Application for grant or renewal of a licensee to repack for sale or distribution of drugs, being drugs other than those specified in Schedule C and C (1) excluding those specified in Schedule X. |
| 16 | Form 24C | Application for the grant or renewal of a license to manufacture for sale [or for distribution] of Homoeopathic medicines or a license to manufacture potentized preparations from back potencies by licensees holding a license in Form 20-C |
| 17 | Form 24D | Application for the grant/renewal of a license to manufacture for sale of Ayurvedic/ Siddha or Unani drugs |
| 18 | Form 24E | Application for grant or renewal of a loan license to manufacture for sale Ayurvedic (including Siddha) or Unani Drugs |
| 19 | Form 24F | Application for grant or renewal of a license to manufacture for sale or for distribution of drugs specified in Schedule X and not specified in Schedule C and C(1). |
| 20 | Form 27 | Application for grant or renewal of a license to manufacture for sale or for distribution of drugs specified in Schedule C and C (1) excluding those specified in part XB and Schedule X |
Conclusion
By obtaining the appropriate license and adhering to regulatory requirements, the pharmaceutical industry can ensure the safe and ethical distribution of medicines in India. Pillsbee can make the online application process less stressful for you by offering free assistance and covering the application fee for your new drug license.
To know more about Pillsbee read – Pillsbee Credit
Frequently Asked Questions
1. Eligibility for acquiring a drug license
To sell medication in both retail and wholesale establishments, it is necessary to have a technical staff member or pharmacist who meets specific qualifications.
- For retail drug license: A B Pharma/DPharma degree holder who is registered with the State Pharmacy Council is required, and prior experience is not necessary.
- For Wholesale drug license: A B Pharma/DPharma degree holder who is registered with the State Pharmacy Council is also necessary, along with either a graduate with at least one year of experience in drug sales or an undergraduate with at least four years of experience in drug sales.
.
2 How much does it cost to open a pharmacy
If you are considering establishing a small-scale pharmacy unit in India, you may need an investment of approximately ₹15 to 25 lakhs. This includes the initial inventory investment, permit, license, rent, staffing, and other overhead costs. According to the scale of the pharmacy unit, the cost will also vary.
3 How to renew a pharmacy license?
Procedure for pharmacy license renewal
- complete the renewal application form which can be downloaded from the official website of the respective state pharmacy council.
- Submit the renewal application form along with the necessary documents. You can either submit your renewal application online or on the other hand in person at the state pharmacy council office.
- submitted your renewal application, the state pharmacy council will review it and may examine the premises.
- If approved, you should receive your renewed license.
Necessary documents
4. How to get a wholesale drug license in India?
Go to the respective state’s drug controlling authority website.
- Download or fill out the application form for a drug license online on the drug controlling authority website.
- Upload or submit the required documents along with the application form and pay the required fees.
- An inspector will visit the premises or shop and verify all the documents and the correctness of the filled data.
- After the verification, the drug controller issues the drug license
5. Can I open multiple Retail pharmacy stores in different states?
Yes, To open multiple retail pharmacy stores in different states, you must apply for individual licenses for each store. Additionally, you must register with the respective state pharmacy council in each state. It is essential to have at least one certified pharmacist per store. Please note that you can use the same store name for both locations.
6. How much does it cost to obtain a drug license?
When applying online, the fee for the drug license should be paid in the government treasury or government branch of India of the district where the license is required. The challan should be submitted along with the application.
| Type of drug license | Fee for grant |
| Retail Licence | 1500 + 1500 + 2%. of the license fee for every month |
| Wholesale Licence | 1500 +1500 +2%. of the license fee for every month |
| Restricted license | 500 +500 +2%. of the license fee for every month |
| Retail license for Drugs specified in Schedule ‘X’ | 500 +500 +2%. of the license fee for every month |
| Wholesale Licence for Drugs specified in Schedule ‘X’ | 500 +500 +2%. of the license fee for every month |
